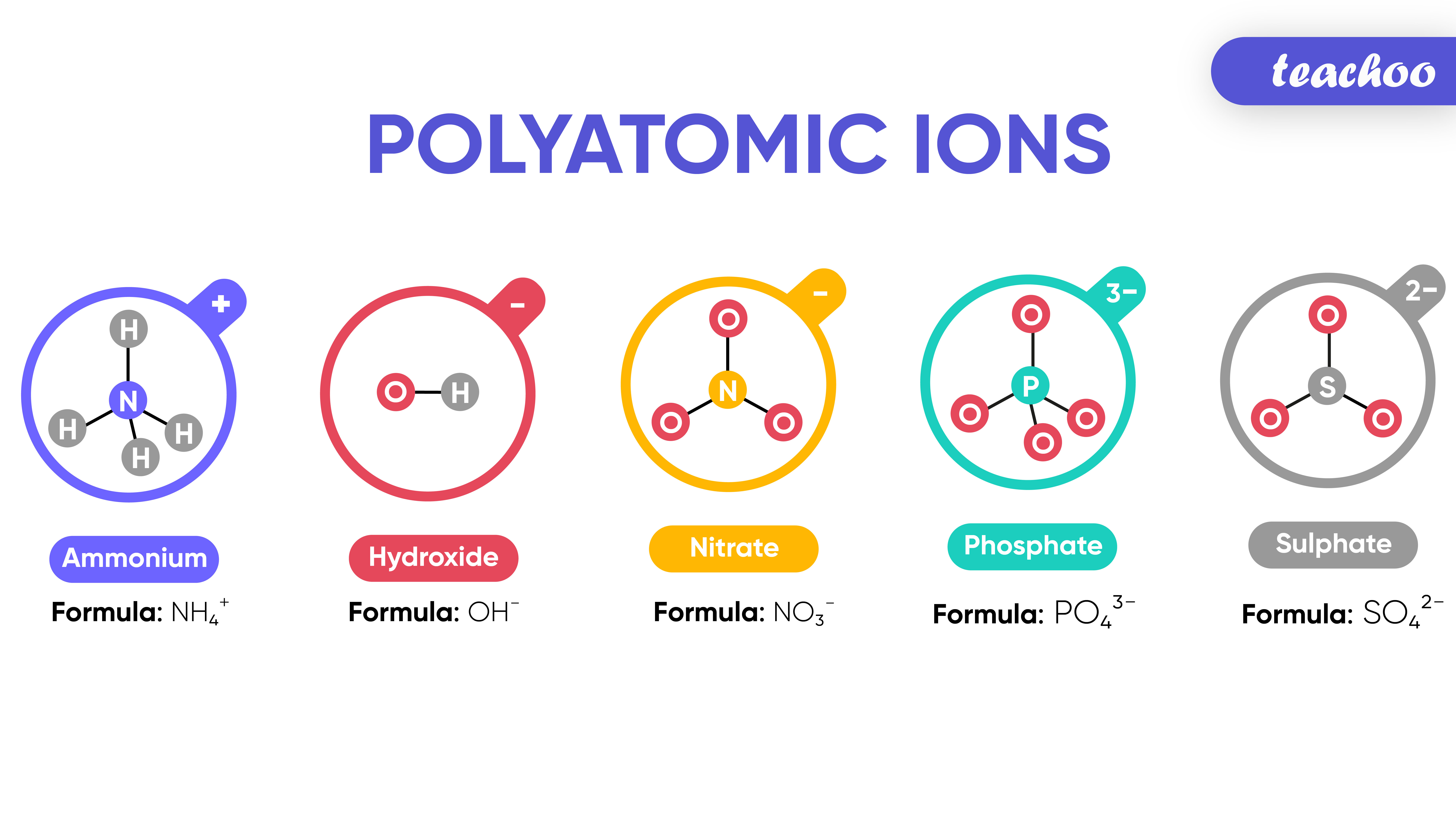
Neat Tips About How To Write Formulas For Polyatomic Ions
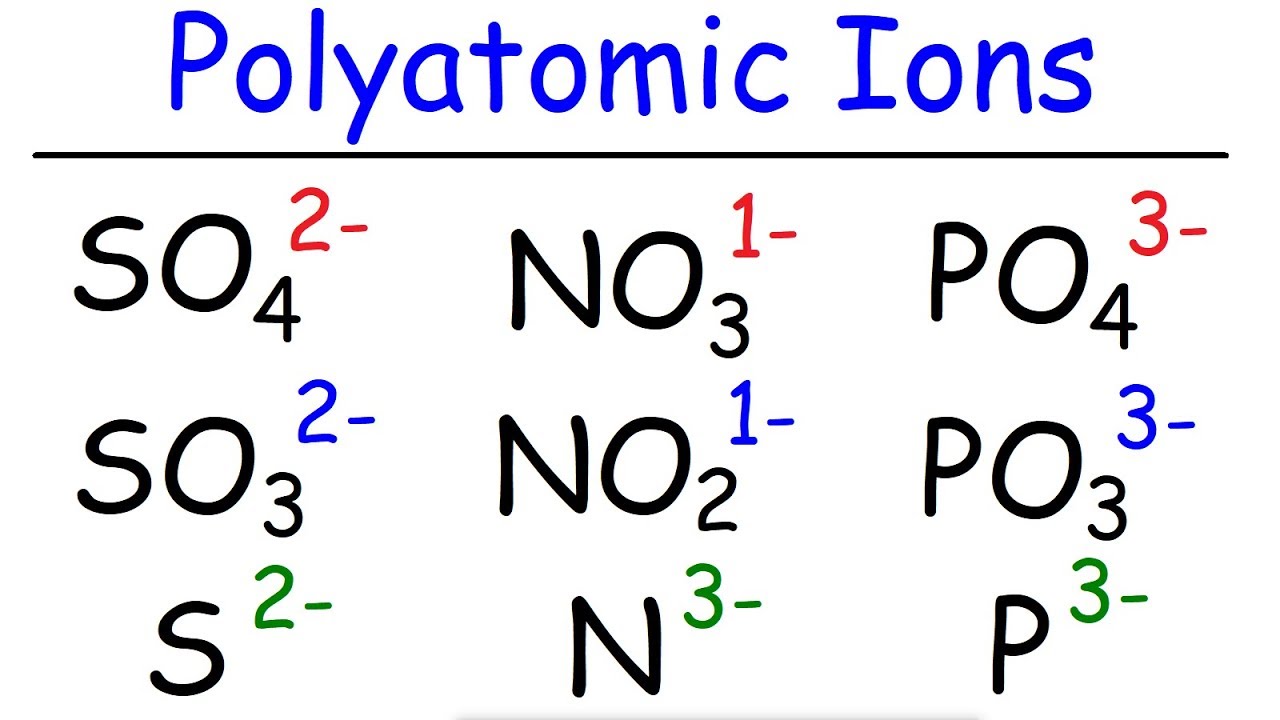
After giving you a simple explanation we look at a couple exampl.
How to write formulas for polyatomic ions. This chemistry video explains the process of writing chemical formulas for ionic compounds with polyatomic ions, transition metals and roman numerals. Write the chemical formula for an ionic compound and name them. Summarize any video by yourself.
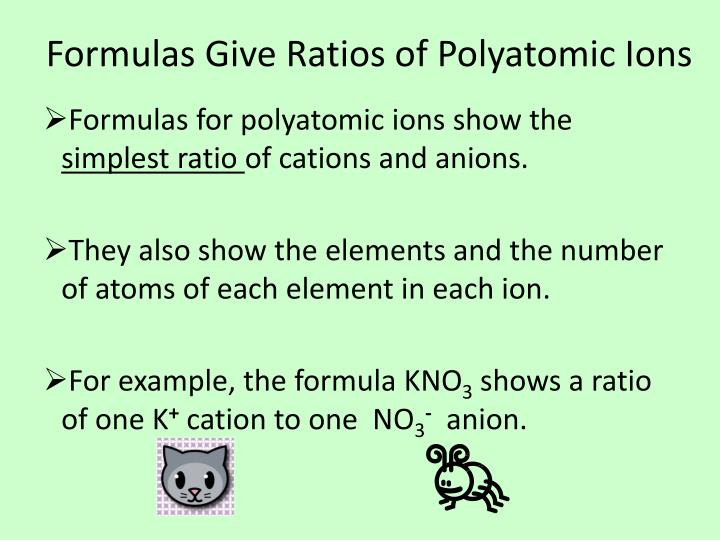
Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary. In this video we'll write the correct formulas for ionic compounds with polyatomic ions (ionic compounds with three different elements). Learn about writing the chemical formula for ionic compounds.
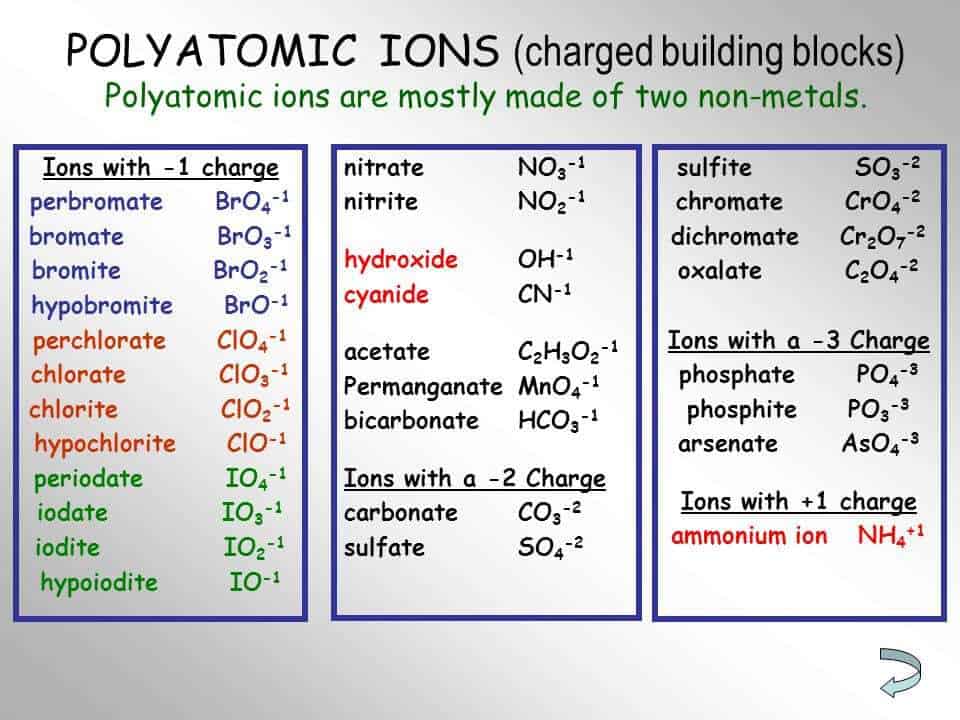
Writing formulas with polyatomic ions. When writing formulas for compounds that have polyatomic ions, you need to use a list of common ions. Writing formulas for ionic compounds containing polyatomic ions.
A chemical formula is a. When there are two or more polyatomic ions in a. Except when there is more than one polyatomic ion, then its.
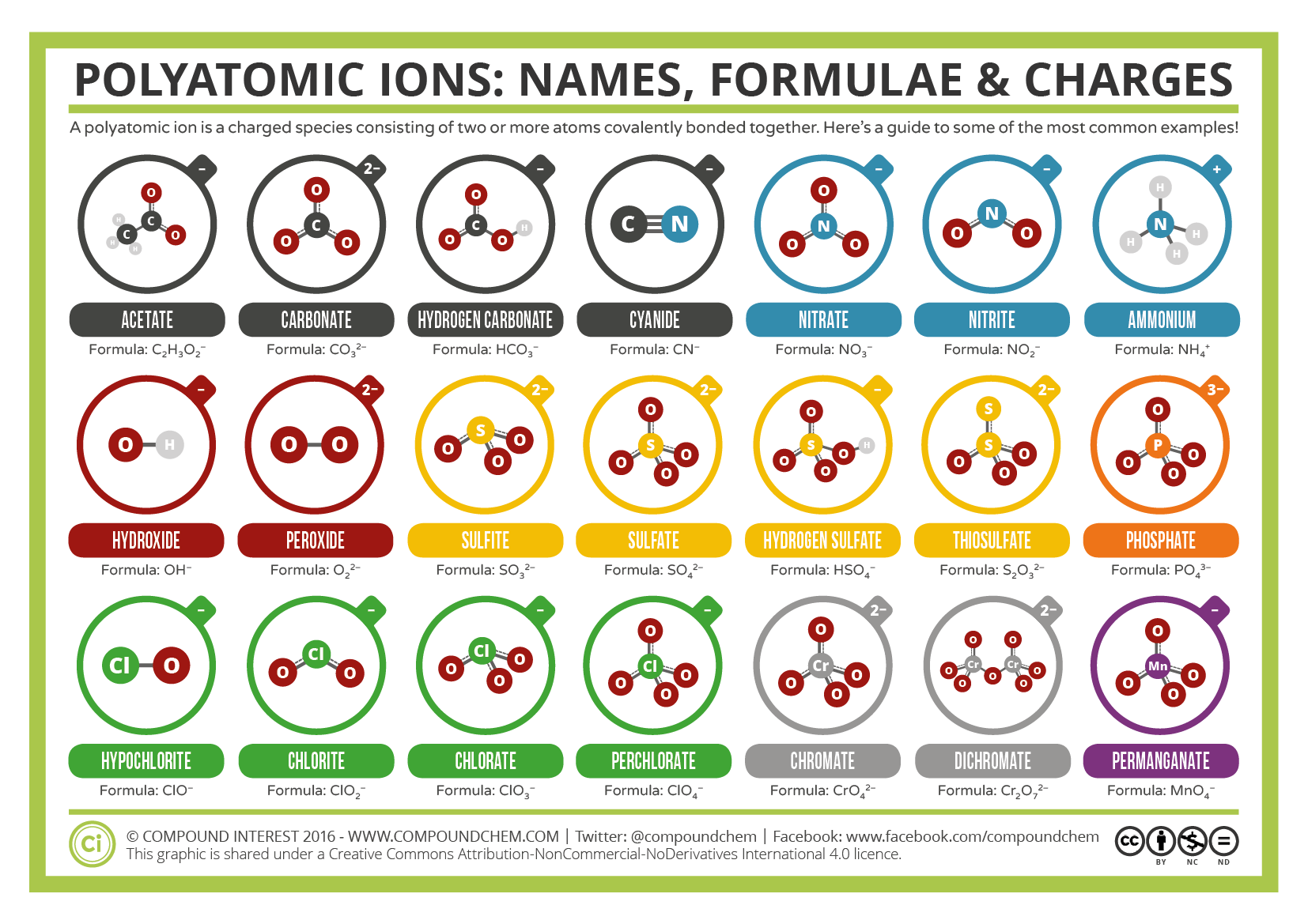
Learn what polyatomic ions are and how they bond. What are 3 examples of polyatomic ions? Writing ionic compounds example 1.
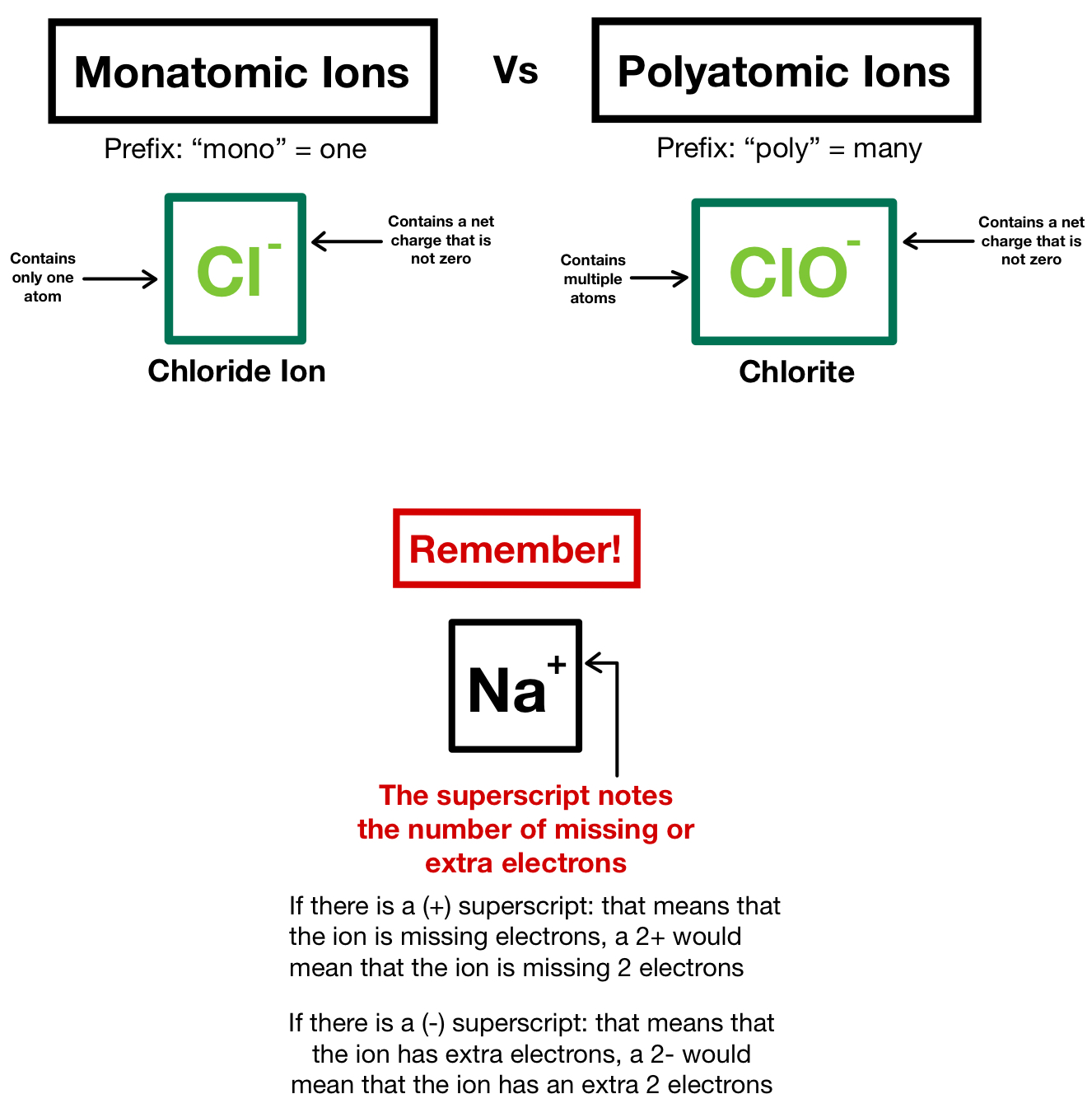
Writing the formulas of ionic compounds has one important difference. Some ions consist of a single atom with a net charge. When you write the formula for an ionic compound, remember that the positive ion is always listed first.
This list is usually given to you by your instructor. We have already encountered some chemical formulas for ionic compounds from monoatomic ions. When writing the formula for an ionic compound, if the charges of the ions are the same, you can simply write the elements.
Containing polyatomic ions are worked out in a similar way to single atom ions. In compounds formed with polyatomic ions, the number of ions present in the compound is indicated by enclosing the formula of ion in a bracket and writing the. The positive ion (cation) is written first in the name;
When the formula unit contains two or more of the. How to draw lewis structures. If more than one polyatomic ion is needed to balance the overall charge in the formula,.
The negative ion (anion) is written second in the name. 0:00 / 10:40. Instructor elizabeth (nikki) wyman view bio.